electronic configuration of cr 3|Chemistry of Chromium : Pilipinas Potassium (K) - Electron Configuration for Chromium (Cr, Cr2+, Cr3+) Those looking for great welcome offers can get £20 in free bets at Coral by signing up and making a £5 qualifying bet. Our Coral Review. Coral is a long-established brand with retail betting as well as online betting and games. As someone who visits and uses a lot of online sportsbooks, it’s evident that Coral is owned by the same company .[Updated September 2, 2024] 4-DIGIT LOTTO RESULTS today. 4D Lotto results draws are posted every Monday, Wednesday and Friday at 9pm. Below is the recent 4-Digit lotto result with tips released .
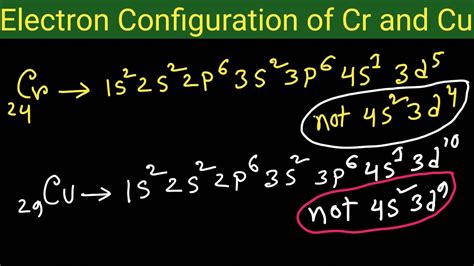
electronic configuration of cr 3,How to Write the Electron Configuration for Chromium (Cr, Cr2+, and Cr3+) In order to write the Chromium electron configuration we first need to know the number of electrons for the Cr atom (there are 24 electrons). Once we have the configuration for Cr, the ions are .
Copper - Electron Configuration for Chromium (Cr, Cr2+, Cr3+)Nitrogen (N) - Electron Configuration for Chromium (Cr, Cr2+, Cr3+)In order to write the Mg electron configuration we first need to know the .Potassium (K) - Electron Configuration for Chromium (Cr, Cr2+, Cr3+)In order to write the Na electron configuration we first need to know the .Lithium - Electron Configuration for Chromium (Cr, Cr2+, Cr3+)Boron is the fifth element with a total of 5 electrons. In writing the electron .The electronic configuration of chromium is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 5. Cr 3 + is formed by losing three electrons from the neutral chromium atom. So, the electronic . To write the configuration for the Chromium ions, first we need to write the electron configuration for just Chromium (Cr). We first need to find the number of electrons for the Cr atom. Mar 23, 2023 Two of these essential trace elements, chromium and zinc, are required as Cr 3+ and Zn 2+. Write the electron configurations of these cations. Solution. First, write the electron configuration for the neutral atoms: .Chemistry of Chromium Cr's electron configuration, following the model would be: \(1s^2 2s^2 2p^6 3s^2 3p^6 4s^23d^4\0, but instead it is \(1s^2 2s^2 2p^6 3s^2 3p^6 4s^13d^5\), because .
It includes: reactions of chromium (III) ions in solution (summarised from elsewhere on the site); the interconversion of the various oxidation states of chromium; . The electron configuration of chromium is [Ar]3d54s1. Explanation: The typical energy level diagram you see in text books showing the 4s below the 3d is ok up to calcium. After that the 3d sub - .
electronic configuration of cr 3 The electronic configuration of Cr 3+ is 1S 2 2S 2 2P 6 3S 2 3P 6 3d 3 4S 0. The number of electrons in the Cr atom is 24, and its outermost atomic orbitals are 4s 1 3d 5. So removed an electron from the 4 orbital and 2 electrons from the 3d-orbital to get the Cr 3+ electronic configuration.
The electronic configuration of chromium is : The atomic number of chromium is 24. Its electronic configuration in ground state is 1s2 2s2 2p6 3s2 3p6 4s1 3d5. Chromium atom by losing 3 electrons form Cr3+ ions. A chromium atom contains 17% more neutron than the protons. Now answer the following questions.
The atomic number of Cr is 24. There are 6 electrons in the outer two shells of Cr that is, 3d and 4s. The assumed electronic configuration of Cr is [Ar]3d 4 4s 2 . Filling of electron take place in increasing order of n + l value of orbital. For 3d orbital, Substitute, 3 for n and 2 for l thus, n + l = 3 + 2 = 5

Cr's electron configuration, following the model would be: \(1s^2 2s^2 2p^6 3s^2 3p^6 4s^23d^4\0, but instead it is \(1s^2 2s^2 2p^6 3s^2 3p^6 4s^13d^5\), because there is extra stability gained from the half-filled d orbital. . Write the ground state electron configuration for P 3-Solution 1. Expanded: 1s 2 2s 2 2p 6 3s 2 3p 5. Shorthand .The chemical symbol of Chromium is ‘Cr’ Electronic configuration of d-block. In general, the outer electronic configuration of these elements is (n-1) d 1-10 n s 1-2. Here, (n–1) stands for the inner d orbitals which may have one to ten electrons, and the outermost ns orbital may have one or two electrons. Electronic configuration of Cr Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Electron configuration of Chromium (Cr) [Ar] 3d 5 4s 1: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5 4s 1: 2, 8, 13, 1: 25: Electron configuration of Manganese (Mn) [Ar] 3d 5 4s 2: 1s 2 2s 2 .Write down the number of 3d electrons in each of the following ions: Ti , V , Cr , Mn , Fe , Fe , CO , Ni and Cu . Indicate how would you expect the five 3d orbitals to be occupied for these hydrated ions (octahedral). Write down the electronic configuration of Cr 3+P m 3+Cu +Ce 4+Co 2+Lu 2+Mn 2+Th 4+.
The answer is [Ar] 4s1 3d5Because half-filled subshells are somewhere stable, the atom prefers to have a half-filled 4s and half-filled 3d's than a full 4s a.For the electronic configuration of the Cr 3+ ion, we will remove three electrons from the Cr (one from the 4s 1 and two from the 3d 5), leaving us with 1s 2 2s 2 2p 6 3s 2 3 6 3d 3 or [Ar] 3 d 3. Unacademy is India’s largest online learning platform. Download our apps to .
In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x. The energy level, "n", can be determined based on the periodic table, simply by looking at the row number in which the element is in. However, there is an exception for the d-block and f-block, in which the energy level, "n" for the d block is . The electronic configuration of Cr(24) atom is: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 5 which is half-filled d-orbital. Cr 3+ has 3 electrons removed from the outermost shell. Therefore, the electronic configuration comes out to be [Ar]3d 3.In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic .
Write down the electronic configuration of: (i) Cr3++ (iii) Cu+ (v) Co2+ (vii) Mn2+ (ii) Pm3+ (iv) Ce4+ (vi) Lu2+ (viii) Th4+ . Prev Question Next Question →. 0 votes . 23.7k views. asked Dec 22, 2017 in Chemistry .
This page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. For phosphorus (element 15) as an example, the concise form is [Ne] 3s 2 3p 3.The stability of oxidation state depends mainly on electronic configuration and also on the nature of other combining atom. The elements which show largest number of oxidation states occur in or near the middle of series (i.e., 4s 2 3d 3 to 4s 2 3d 7 configuration). For example, Mn exhibits all oxidation states from +2 to +7 as it has 4s 2 3d 5 .We can summarize this for the complex [Cr(H 2 O) 6] 3+, for example, by saying that the chromium ion has a d 3 electron configuration or, more succinctly, Cr 3 + is a d 3 ion. Figure \(\PageIndex{2}\): The Possible Electron Configurations for Octahedral d n Transition-Metal Complexes (n = 1–10).
52 Cr: Electron configuration [Ar]3d 5 4s 1: CAS number: 7440-47-3 : ChemSpider ID: . The electron configuration of chromium is [Ar]3d 5 4s 1, which can be explained by the stability offered by a half-filled d-orbital. Trivalent chromium is a vital nutrient that is found in traces of sugar, human insulin, and lipid metabolism.electronic configuration of cr 3 Chemistry of Chromium Write the electronic configuration of (i) Mn4+, (ii) Fe3+ (iii) Cr2+ Mention the number of unpaired electrons in each case.
electronic configuration of cr 3|Chemistry of Chromium
PH0 · What is the electron configuration of chromium?
PH1 · What is the electron configuration of Cr 3+? Chemistry Q&A
PH2 · What is the electron configuration of Cr 3+? Chemistry Q&A
PH3 · What is the electron configuration of Cr 3+?
PH4 · What is the electron configuration of Cr
PH5 · The electronic configuration of { Cr }^{ 3
PH6 · Electron Configuration for Cr, Cr2+, and Cr3+ (Exception to Rules)
PH7 · Electron Configuration for Chromium (Cr, Cr2+, Cr3+)
PH8 · Electron Configuration for Chromium (Cr and Cr2+, Cr3
PH9 · Electron Configuration Chart of All Elements (Full Chart)
PH10 · Chromium (Cr)
PH11 · Chemistry of Chromium
PH12 · 3.1: Electron Configurations
PH13 · 1.9A: Ground State Electronic Configurations